pH
pH is a measurement of how acidic or basic water is, indicating the solubility (amount of chemicals that can be dissolved in water) and organic accessibility (amount of dissolved chemicals available to life) of chemical components in water. These chemicals include nutrients like nitrogen, phosphorous, and carbon, as well as heavy metals like iron, zinc, copper, etc. Measured on a scale from 0 to 14, with 7 being neutral, a pH from 0 to 7 indicates acidity, with more free H ions in the water, while a pH of 7 to 14 indicates a base, with more free OH ions. Remember, water is H2O, which can be broken down into an H and an OH, as seen in the equation: H2O (l) ⇌ H+(aq) + OH-(aq). Here, (l) means that the water is liquid, and (aq) means that the ions are in an aqueous (water-based) solution; pH measures the relative concentration of each of these breakdowns in solution.
Common substances and their typical pH. The scale ranges from 0, most acidic, to 14, most basic, with water having an average pH of 7. To the left of the scale indicates biological impacts of increasing acidity, with rainbow trout beginning to die at pH of 6.0, frog eggs, tadpoles, crayfish, and mayflies at 5.5, and all fish dying at 4.2 and below.
Image source: EPA
pH values on either end of the spectrum, excessively high or low, can have significant negative impacts on water use. High pH values often result in deposits on water pipes and require additional chlorine to adequately disinfect pipes and appliances. On the flip side, low pH water has increased corrosive and dissolving capabilities. Biologically, most organisms require a fairly narrow, neutral pH in and outside their bodies, with more extreme pH environments requiring special adaptations for life, as is commonly seen in archaea and even stomach bacteria!
Aquatic pH levels with corresponding biologic impacts. Optimum pH values for humans exist between 4.0 and 11.0, plants between 6.2 and 12.5, and fish between 6.5 and 9.0. Fish die below a pH of 4.2 and above 10.2.
Image sourced from Fondriest Environmental, Inc. “pH of Water.” Fundamentals of Environmental Measurements. 19 Nov. 2013.
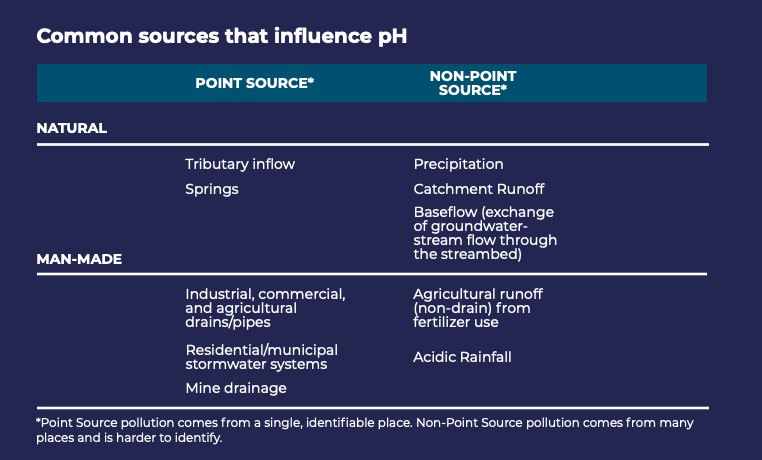
The pH of water is influenced by many different factors, including soil composition, organic material, human activities (e.g., mining, agricultural runoff, industrial emissions), and precipitation.
Soil Composition: Water moves through soil and bedrock as both surface and groundwater; thus, the composition of these materials affects the pH of the water itself. Rocks like limestone can neutralize water (make it less acidic) to a degree, whereas rocks such as basalt, gneiss, granite, etc have little impact on water’s pH.
Organic Material: Water’s interaction with biotic (living) components affect it’s chemistry in a multitude of ways- significantly relating to pH is the formation of carbonic acid, a weak acid. When organic material decomposes within a body of water, it releases carbon dioxide, which combines with water to form carbonic acid. While weak, carbonic acid in large amounts will lower pH.
Human Activity- Mining: The mineral iron sulfide (FeS2), found commonly in and around coal seams (banded deposits of coal visible within rock), has been found to significantly increase acidic mine runoff. When exposed to air and water, FeS2 forms iron(III) hydroxide, Fe(OH)3, which precipitates out of solution (becomes a solid from the liquid solution), and sulfuric acid, H2SO4. Because the solubility (read: dissolvability) of Fe(OH)3 depends on the temperature and pH of the water, variable levels of Fe(OH)3 will precipitate out when the acidic solution mixes with river water. Therefore, not only will H2SO4 significantly lower pH, the amount of dissolved Fe(OH)3 will significantly impact water chemistry.
Human Activity- Dumping: Dumping chemicals into water- by individuals, communities, and corporations- have a significant impact on the pH of a body of water. It’s easy to conjure the image of large-scale corporate dumping of toxic chemicals, which certainly significantly impacts water health. Especially since many industries will introduce chemicals into their water to adjust it’s pH to their needs before discharging it either directly into a body of water or via treatment plant. But even a load of laundry or shampoo rinse can impact water composition in ways beyond just pH. Think of it this way: water is composed of partially charged H2O molecules whose job is to form bonds. Add any other chemical to this mix and suddenly you have a chance for chemical reaction to occur, changing what was once pure H2O into a collection of various solutes and solvents. This is obviously an oversimplification, but a quick look at how many things in our world are water-based, or in aqueous solution, shows water’s fundamental role in chemical reactions.
Precipitation: The amount of acid precipitation that falls in a watershed will impact the pH of the water, as seen in the diagram below:
The pathways involved in creating acid rain.
Image sourced from EPA
Gaseous nitrogen oxides (NOx) and sulfur dioxide (SO2) from automobile and power-plant emissions are released into the air (1) and are transported long distances (2). These particles in the atmosphere then interact with water vapor, forming what is known as “acid rain”, that is precipitated, or return to earth as dry deposition (3). As this acid rain precipitates into soils and seeps downwards, it dissolves other minerals that change the chemistry of the resulting ground and surface water, causing harmful effects on many different ecosystems (4). Not only does this force streams towards becoming more acidic, when spring snowmelt comes, streams can also receive a huge dose all at once as acidic snows melt.
Common sources influencing pH.
Sources and Resources:
On pH:
Acids, Bases, pH, and Buffers from Khan Academy
pH and Water from USGS Water Science School
pH in the Environment Ecosystem from Water Research Center
Life in pH extremes:
Life in Extreme Environments by Rothschild, L., Mancinelli, R.
The hunt for living gold. 2001 European Molecular Biology Organization.